Press Release
Johns Hopkins APL Devises Clean, Cost-Effective Method to Eliminate PFAS ‘Forever Chemicals’ in Water
The great irony of nonstick coatings is that they actually do stick around — forever. Now, scientists at the Johns Hopkins Applied Physics Laboratory (APL) in Laurel, Maryland, have devised a unique way to destroy these ubiquitous and toxic environmental chemicals for good. The method is inexpensive, does not generate undesirable byproducts and is practical and scalable for a variety of governmental, civilian and commercial applications.
PFAS, or perfluoroalkyl and polyfluoroalkyl substances, are a group of some 5,000 artificial chemicals commonly found in nonstick and stain-resistant coatings, like those used in cookware, carpets, outdoor gear and food packaging, as well as in firefighting foams and a plethora of other industrial applications. They are also commonly found in water — and consequently also in wildlife and human beings. Their tendency to linger indefinitely in the environment without degrading has earned PFAS the label “forever chemicals.”
The known and suspected health dangers of PFAS include thyroid, immune and liver disruption, as well as cancer. So well-recognized are the health risks that $10 billion of the infrastructure bill recently passed by the Senate was allotted to addressing their presence in drinking water.
Most methods developed to deal with PFAS in water have focused on PFAS sequestration via filters or similar technologies. However, short of complete destruction of the hardy molecules, the risk of secondary contamination during filter disposal remains a major concern.
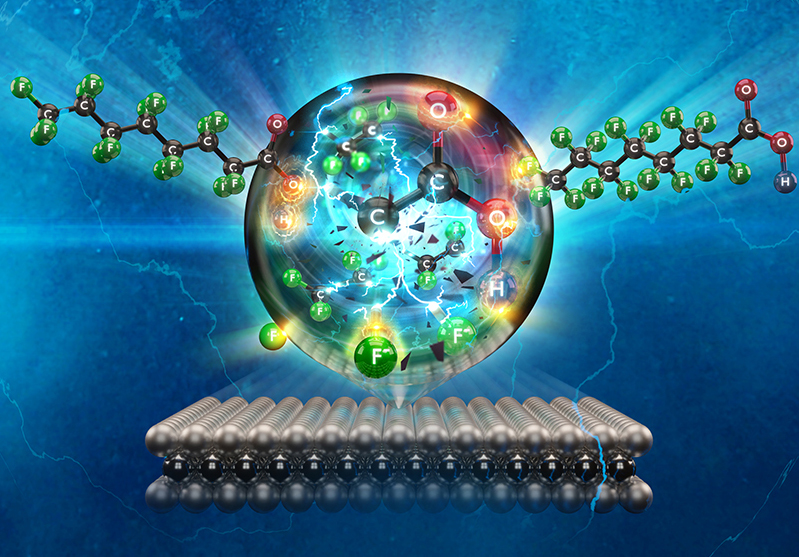
“Breaking down PFAS is not an easy task due to the high bonding energy of carbon-fluorine [C-F] atoms,” said Zhiyong Xia, chief scientist of the Exploratory Science Branch in APL’s Research and Exploratory Development Department (REDD). “We are tackling this problem from a fundamental perspective — that is, cutting the C-F bonds in PFAS, reducing the large molecules into smaller, environmentally benign species.”
The technique developed by APL scientists from REDD and the Asymmetric Operations Sector uses a novel catalyst, made from titanium oxide doped (intentionally impurified) with niobium, to induce an electrochemical reaction, generating free radicals that break the powerful C-F bonds that hold PFAS together.
While the electrochemical approach has been taken by others, the catalyst developed by Zhiyong and colleagues is new and, most importantly, cost-effective. Previous work has relied on catalysts made from diamond, platinum, iridium and other precious materials, which would make implementation prohibitively expensive.
“Our strategy has been to avoid precious materials altogether,” said Xia. “To guide our catalyst design, we rely on atomic-level simulations to identify potential catalysts. After narrowing down possible candidates, we create and test promising catalysts for lab evaluation.”